Solvent Selection:
Solvents are a key area of focus for organic chemists looking to improve the greenness of their processes as they are usually the largest mass input of a synthetic process or transformation.A comprehensive tool for evaluating a solvent or potential alternative is the ACS GCIPR interactive solvent guide (depicted below). This tool allows permits interactive solvent selection based upon the Principal Component Analysis (PCA) of the solvent’s physical properties. Solvents which are close to each other in the map have similar physical and chemical properties, whereas distant solvents are significantly different. In addition, other data including the physical poperties, functional groups, and environmental data has been included to aid nthe rational selection of solvents. This tool is further defined in "Toward a More Holistic Framework for Solvent Selection". Diorazio, L. J.; Hose, D. R. J.; Adlington, N. K., Org. Process Res. Dev. 2016, 20, 760-773.
Solvent Literature:
- "CHEM21's selection guide of classical- and less classical-solvents". Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C. R.; Abou-Shehada, S.; Dunn, P. J. Green Chem. 2016, 18, 288-296. (open access)
- "GSK: Updating and Further Expanding GSK's Solvent Sustainability Guide". Alder, C. M.; Hayler, J. D.; Henderson, R. K.; Redman, A. M.; Shukla, L.; Shuster, L. E.; Sneddon, H. F. Green Chem. 2016, DOI: 10.1039/C6GC00611F.
- "Pfizer: Green chemistry tools to influence a medicinal chemistry and research chemistry based organisation". Alfonsi, K.; Colberg, J.; Dunn, P. J.; Fevig, T.; Jennings, S.; Johnson, T. A.; Kleine, H. P.; Knight, C.; Nagy, M. A.; Perry, D. A.; Stefaniak, M. Green Chem . 2008, 10, 31-36.
- "Sanofi's Solvent Selection Guide: A Step Toward More Sustainable Processes". Prat, D.; Pardigon, O.; Flemming, H.; Letestu, S.; Ducandas, V.; Isnard, P.; Guntrum, E.; Senac, T.; Ruisseau, S.; Cruciani, P.; Hosek, P. Org. Proc. Res. Dev . 2013, 17, 1517-1525.
- "AstraZeneca: Toward a More Holistic Framework for Solvent Selection". Diorazio, L. J.; Hose, D. R. J.; Adlington, N. K. Org. Proc. Res. Dev. 2016, ASAP (open access)
- "NMR Chemical Shifts of Trace Impurities: Industrially Preferred Solvents Used in Process and Green Chemistry (peaks of residual solvents in 6 different NMR solvents)". Babij, N. R.; McCusker, E. O.; Whiteker, G. T.; Canturk, B.; Choy, N.; Creemer, L. C.; De Amicis, C. V.; Hewlett, N. M.; Johnson, P. L.; Knobelsdorf, J. A.; Li, F.; Lorsbach, B. A.; Nugent, B. M.; Ryan, S. J.; Smith, M. R.; and Yang, Q. Org. Process Res. Dev., 2016, 20, 661-667. (open access)
- "Development of GSK's NMR Guides – A tool to encourage the use of more sustainable solvents". Gottlieb, H. E.; Graczyk-Millbrandt, G.; Inglis, G. A.; Nudelman, A.; Perez, D.; Qian, Y.; Shuster, L. E.; Sneddon, H. F.; Upton, R. J. Green Chem. 2016 DOI: 10.1039/C6GC00446F.
- "A convenient guide to help select replacement solvents for dichloromethane in chromatography". Taygerly, J. P.; Miller, L. M.; Yee, A.; Peterson, E. A. Green Chem. 2012, 14, 3020-3025.
- "Replacement of dichloromethane within chromatographic purification: a guide to alternative solvents". MacMillan, D. S; Murray, J.; Sneddon, H. F. ; Jamieson, C.; Watson, A. J. B. Green Chem. 2012, 14, 3016-3019.
- "Development of a tripartite solvent blend for sustainable chromatography". Chardon, F. M.; Blaquiere, N.; Castanedo, G. M.; Koenig, S. G. Green Chem. 2014, 16, 4102-4105.
- Free Green Chemistry MOOC – created by IMI CHEM21 consortium. Due to be launched on 13 th June – but the test version is available to view here: http://test-chem21-elearning.pantheon.io/ . Pharmaceutical Green Chemistry is biased although the information is generalizable to other disciplines or industries

Reagent Selection:
Selecting the most sustainable reagent to use for organic chemistry transformations requires the assessment of many factors including atom efficiency, toxicology, safety, waste products, sustainable feedstocks, and more. Industrial multidisciplinary chemists, as members of the ACS GCIPR, have compiled reagent guides to inform and assist organic chemists in the selection of reagents for >19 transformations or procedures.Each guide, in the collection of guides, is a comprehensive resource composed of:
- A general overview of the transformation
- A Venn diagram representation
- General literature reviews
- Special considerations (eg. safety) and green criteria
- List of reagents
- Mechanism
- Key references
- Scale-up examples
References:
- ACS: Green Chemistry Institute's Pharmaceutical Roundtable
- "Development of GSK's Reagent Guides – Embedding Sustainability into Reagent Selection". Adams, J. P.; Alder, C. M.; Bullion, A. M.; Campbell-Crawford, M.; Darcy, M. G.; Hayler, J. D.; Henderson, R. K.; Oare, C. A.; Pendrak, I.; Redman, A. M.; Shuster, L. E.; Sneddon, H. F.; Walker, M. D. Green Chem. 2013, 15, 1542-1549 .
- "Development of GSK's Acid and Base Selection Guides". Henderson, R. K.; Hill, A. P.; Redman, A. M.; Sneddon, H. F. Green Chem. 2015, 17, 945-949.
- "Evaluation of Alternative Solvents in Common Amide Coupling Reactions: Replacement of Dichloromethane and N, N -Dimethylformamide" MacMillan, D. S.; Murray, J.; Sneddon, H. F.; Jamieson, C.; Watson, A. J. B. Green Chem. 2013, 15, 596-600 (open access).
- "Development of a Solvent Selection Guide for Aldehyde-based Direct Reductive Amination Processes". McGonagle, F. I.; MacMillan, D. S.; Murray, J.; Sneddon, H. F.; Jamieson, C.; Watson, A. J. B. Green Chem. 2013, 15, 1159-1165.


Biocatalysis guide:
Biocatalysis is a key green technology for modern sustainable organic syntheses. The Biocatalysis Guide is a simple double-sided, single-sheet guide to the currently most used enzyme classes amongst the ACS GCI member companies. It has been produced to be an easy-to-follow guide for chemists who have not had significant exposure to biocatalysis, showing generic transformations that are available so these can be factored into retrosynthetic analysis.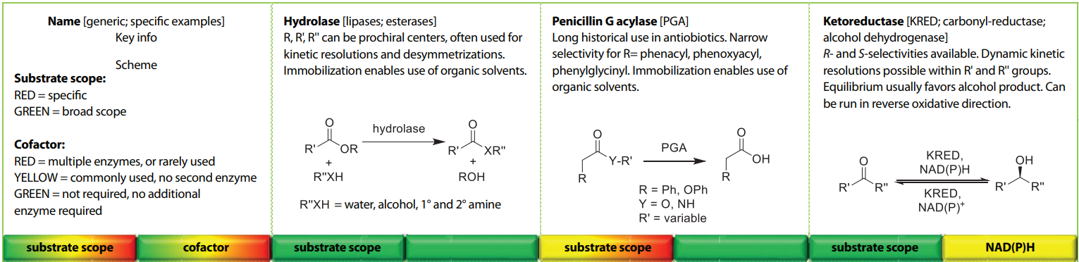
Med Chem:
All parts of pharmaceutical development can be made more sustainable. A great example of this is the ACS GCIPR Medicinal Chemistry Team’s approaches to greening medicinal chemistry. The team produced this quick guide covering purification, solvent selection, reagents, energy and resources.Process Mass Intensity:
Process mass intensity (PMI) is the key green mass-based metric for measuring the resource usage impact of a synthetic chemistry process.$$PMI = \frac{Mass of Raw Materials Input}{Mass of Product}$$
The PMI calculator enables organic chemists to quickly determine the PMI number from the raw material inputs and final product yield. The calculator accommodates multi-step convergent syntheses and includes breakdown of solvent, reagents, and water PMI. These calculations are an invaluable method to drive development of more sustainable processes and to track the mass efficiency for a synthetic procedure.Process mass intensity data has been gathered to provide benchmarking data for the small-molecule, oligonucleotide, peptide, and monoclonal antibody therapeutic classes.
References:
- ACS Sustainable Chem. Eng. 2022, 10, 5148–5162
- mAbs: Budzinski, K.; Blewis, M.; Dahlin, P.; D’Aquila.; Esparza, J.; Gavin, J .; Ho, S.V.; Hutchens, C.; Kahn, D.; Koenig, S.G.; Kottmeier, R.; Millard, J.; Snyder, M.; Stanard, B.; Sun, L.; New Biotechnology, 2019, 49, 37–42
- Oligonucleotides: Andrews, B.I.; Antia, F.D.; Brueggemeier, S.B.; Diorazio, L.J.; Koenig, S.G.; Kopach, M.E.; Lee, H.; Olbrich, M.; Watson, A.L. J. Org. Chem. 2021, 86, 49−61
The PMI prediction tool (depicted below) provides a simple and accessible means of predicting the mass efficiency of proposed synthetic routes. The tool is built from a dataset of nearly two thousand multi-kilo reactions provided by pharmaceutical, biotech, and manufacturing ompanies via the ACS GCIPR as well as extracted from the literature. By defining a sequence of reactions and their corresponding reaction type, it is possible to estimate a plausible PMI for ay proposed or unoptimized organic chemistry route. This ability to virtually screen different rspective routes for efficiency allows organic chemists to focus their resources on a few poising synthetic approaches.
This process is elaborated in "The PMI Predictor app to enable green-by-design chemical synthesis". Borovika, A.; Albrecht, J.; Li, J.; Wells, A. S.; Briddell, C.; Dillon, B. R.; Diorazio, L. J.; Gage, J. R.; Gallou, F.; Koenig, S. G.; Kopach, M. E.; Leahy, D. K.; Martinez, I.; Olbrich, M.; Piper, J. L.; Roschangar, F.; Sherer, E. C.; Eastgate, M. D. Nature Sustainability. 2019 , 2, 1034–1040

Green Chemistry Innovation Scorecard Calculator:
Green Chemistry Innovation Scorecard Calculator is a slightly different approach to accounting for PMI by focusing on waste. A joint effort by the IQ Consortium, ACS GCI Pharmaceutical Roundtable, and academic leaders, this Green Chemistry Innovation Scorecard web calculator illustrates how green chemistry and engineering innovation can reduce waste mass during bulk active pharmaceutical manufacture. The calculator uses a statistical analysis of 64 bulk active pharmaceutical manufacturing processes encompassing 703 steps across 12 companies to provide a relative process greenness score. This score may then be used as a means of making meaningful comparisons between different processes and their associated waste reductions.References:
- ACS Sustainable Chem. Eng. 2022, 10, 5148–5162
- "Using the Right Green Yardstick: Why Process Mass Intensity Is Used in the Pharmaceutical Industry To Drive More Sustainable Processes". Jimenez-Gonzalez, C.; Ponder, C. S.; Broxterman, Q. B.; Manley, J. B. Org. Process Res. Dev. 2011, 15, 4, 912–917
General Resources:
- Beyond Benign green chemistry education
- The CHEM21 metrics toolkit
- ACS: Green Chemistry MedChem Tips and Tricks
- Wikipedia's Green Chemistry
- Wikipedia's Green Chemistry Metrics
- Green and Sustainable Medicinal Chemistry: Methods, Tools and Strategies for the 21st Century Pharmaceutical Industry edited by Louise Summerton, Helen F. Sneddon, Leonie C. Jones and James H. Clark; book published by Royal Society of Chemistry, 2016 DOI: 10.1039/9781782625940
- Green Chemistry Strategies for Drug Discovery edited by Emily A. Peterson, Julie B. Manley, book published by Royal Society of Chemistry, 2015 DOI: 10.1039/9781782622659