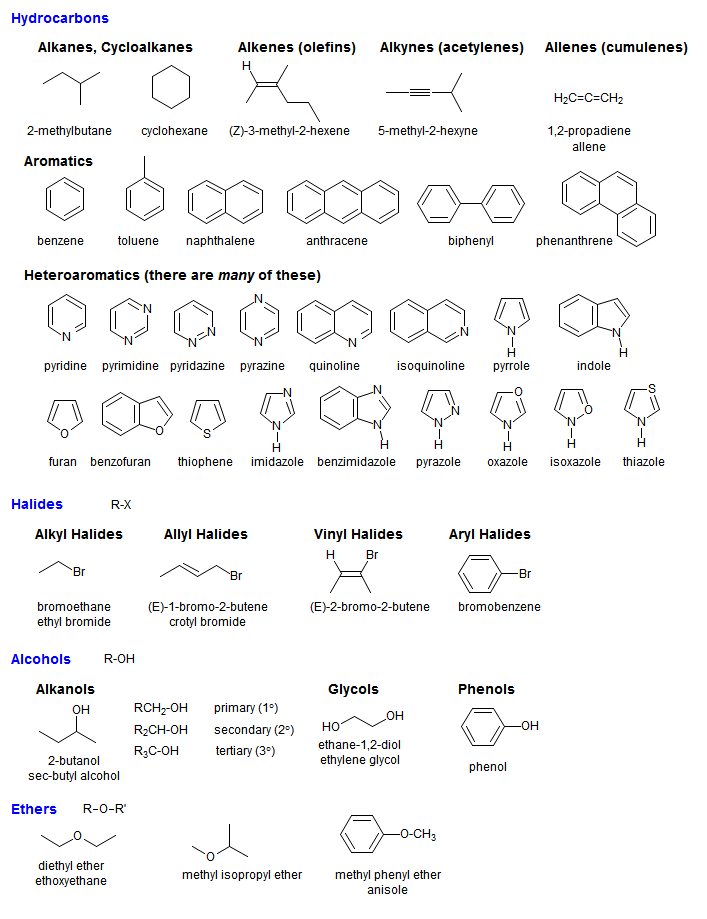
Organic Nomenclature - One Word or Two?
The question of whether the name of an organic compound should be written in one word or two words is a perennial one. Here is a brief summary which should help keep things straight:
Substitution nomenclature takes a parent compound and identifies substituents which replace hydrogens on it with either prefixes (chloro-, phenyl-) or postfixes (-ol, -one). This system can be based on trivial names for the parent compound (chloroacetone) or on names based on systematic rules such as those developed by IUPAC or Chemical Abstracts. Such names are one word, except for certain functional groups (notably acid derivatives like esters and acid chlorides) which retain their ancient two-word salt names.
Salt nomenclature was adopted from inorganic chemistry. In such names the "cation" and "anion" are always separate words, first the cation, then the anion (e.g.: sodium chloride, lithium hydride, cesium carbonate). If the last word ends in "ate" or "ide" you are using salt nomenclature, and your name will be at least two words (e.g.: butyl bromide, allyl iodide). Each of the words will be a radical name, not a compound name.
You should avoid using salt nomenclature for organic compounds that are not ethers, actual salts or derivatives of acids if convenient substituent names are available. However, many salt names are so firmly entrenched and convenient that they are almost second nature (allyl chloride vs 1-chloro-2-propene, or benzyl bromide vs (bromomethyl)benzene or alpha-bromotulene).
Substituent Nomenclature - One Word Salt Nomenclature - Two Words trimethylchlorosilane trimethylsilyl chloride tributylstannane tributylstannyl hydride iodobenzene phenyl iodide dichloromethane methylene chloride 1,2-dibromoethane ethylene dibromide 2-propen-1-ol allyl alcohol (alcohol = hydroxide) 1,2-oxidopropane propylene oxide 1-azidopropane propyl azide Amine is considered a variant of ammonia (analogous to phosphine). Thus amine names use substituent nomenclature, and are always one word. Ether names, on the other hand, are adapted from salt nomenclature (ether = oxide), and each radical is a separate word. (You can't expect a system developed by tens of thousands of chemists over 150 years to be totally consistent). Sulfide, selenide, alcohol, mercaptan, sulfoxide, sulfone, ketone, etc are also treated as salt names, since none of these words describes a specific compound.
Substituent Nomenclature - One Word Salt Nomenclature - Two or more Words triethylamine - triphenylphosphine - ethyldimethylamine - methanol methyl alcohol (alcohol = hydroxide) methoxymethane dimethyl ether (ether = oxide) methoxybenzene methyl phenyl ether benzenethiol phenyl mercaptan 2-butanone methyl ethyl ketone (methylsulfonyl)benzene methyl phenyl sulfone Carboxylic acid derivatives (as well as derivatives of other families of acids such as sulfonic, carbonic, phosphonic, etc) are named as salts. Note that the acids themselves are written as two words (acetic acid, ethanoic acid, methanesulfonic acid).
Salt Nomenclature of Acid Derivatives - Two Words ethyl acetate dimethyl phthalate trimethyl phosphate benzoyl chloride 2,5-difluorobenzyl 2,4,6-trimethylbenzoate methyl benzenesulfonate Element names are usually handled using substituent nomenclature, although things get complicated here because this is not systematic ("Zinc" is not ZnH2, so we are not substituting hydrogens). Inorganic chemists have developed their own usages here (as, incidentally, have other languages).
Substituent Nomenclature - One Word Salt Nomenclature - Two Words diethylzinc - diethylchloroaluminum diethylaluminum chloride methyllithium lithium methide tributylstannylsodium sodium tributylstannate (diisopropylamino)lithium lithium diisopropylamide A very general and useful rule: if the last part of your name is an actual compound then you are using substituent nomenclature (e.g., benzene, methane, butane, biphenyl, stannane, phosphine are compounds). Your name will be be one word. If the last part is not, then you are probably using salt nomenclature (e.g. acetate, chloride, ketone, sulfide, ether, sulfoxide are not the names of compounds, and so substituent nomenclature is not appropriate). Salt names are always two or more words.
Functional Groups Summary
A Functional Groups Summary is available as a PDF file.
Nitrogen-Containing Functional Groups Summary
A Nitrogen-Containing Functional Groups Summary is available as a PDF file.


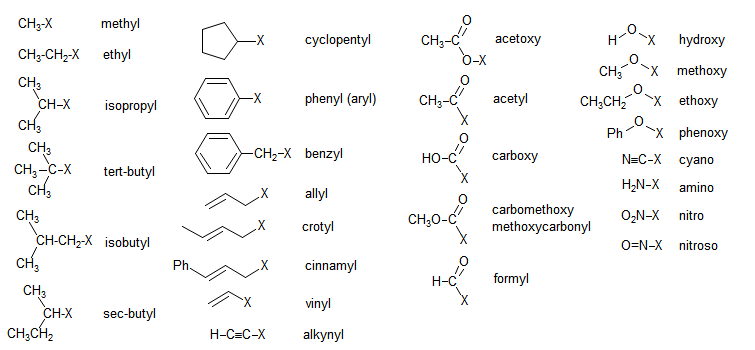
Substituent Groups